Clinical features and survival analysis in acute myeloid leukemia patients with SRSF2 gene mutation
-
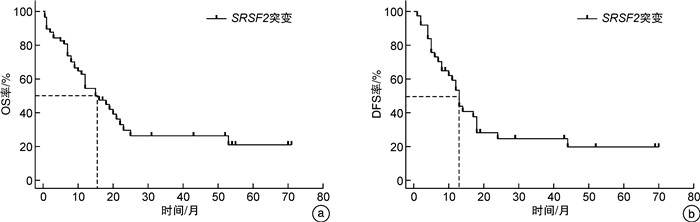
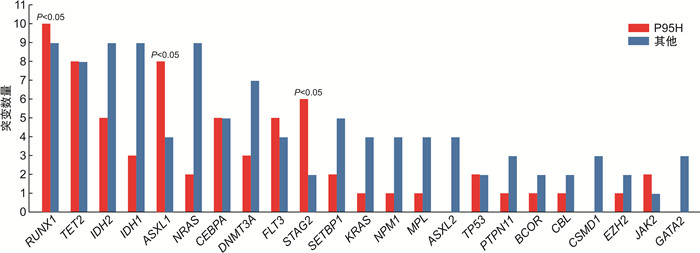
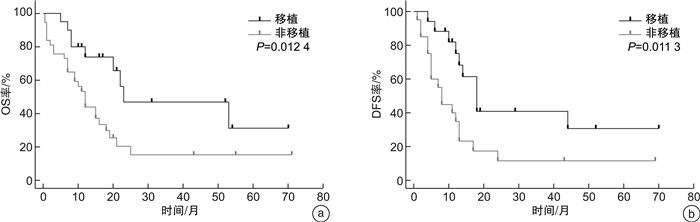
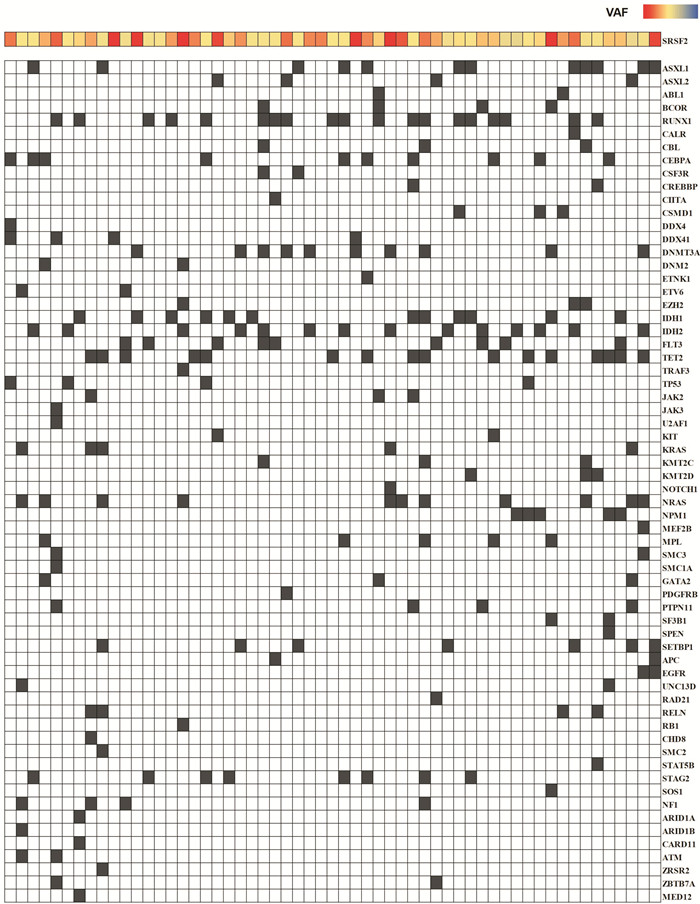
摘要: 目的 研究伴SRSF2基因突变初诊急性髓系白血病(AML)患者的临床特征及异基因造血干细胞移植(allo-HSCT)的疗效。方法 回顾性分析2016年6月—2022年12月就诊于苏州大学附属第一医院且二代测序检测出SRSF2突变的AML患者的临床资料。结果 共57例SRSF2基因突变患者纳入本研究,其中男42例(73.7%),女15例(26.3%),中位年龄60(17~83)岁。57例SRSF2突变患者中,18例(31.6%)患者为SRSF2 P95H突变,15例(26.3%)患者为SRSF2 P95L突变,8例(14.0%)患者为SRSF2 P95R突变。SRSF2 P95H突变与年龄较大、白细胞计数增高、较高的VAF值相关。共突变基因分析显示,SRSF2突变的AML最常见的伴随基因突变为 RUNX1 突变(33.0%)。生存分析显示,57例SRSF2突变AML患者的2年总生存(OS)率为29.6%,2年无病生存(DFS)率为24.7%。接受allo-HSCT巩固治疗组患者2年OS率和2年DFS率分别为46.9%和41.0%,化疗组患者2年OS率和DFS率分别为20.4%和11.7%,2组患者间OS(P=0.012 4)和DFS(P=0.013 3)比较差异均有统计学意义。多因素分析结果显示,年龄是影响SRSF2突变AML患者OS(HR=2.385,95%CI 1.074~5.298,P=0.033)和DFS(HR=3.378,95%CI 1.223~9.334,P=0.019)的独立危险因素。结论 allo-HSCT可以改善SRSF2基因突变初诊AML患者预后。年龄是影响伴SRSF2突变的AML患者OS和DFS的独立危险因素。
-
关键词:
- 急性髓系白血病 /
- 基因 /
- SRSF2 /
- 异基因造血干细胞移植 /
- 生存分析
Abstract: Objective To investigate the clinical characteristics and the efficacy of allogeneic hematopoietic stem cell transplantation(allo-HSCT) in acute myeloid leukemia(AML) patients with SRSF2 gene mutation.Methods From June 2016 to December 2022, the clinical data of patients admitted to the First Affiliated Hospital of Soochow University with SRSF2 mutations detected by next-generation sequencing were retrospectively analyzed.Results 57 patients with AML were included in the study, comprising 42 males(73.7%) and 15 females(26.3%), with a median age of 60(17-83) years. Among the 57 patients with SRSF2 mutations, 18 cases (31.6%) had SRSF2 P95H mutations, 15 cases (26.3%) had SRSF2 P95L mutations, and 8 cases (14.0%) had SRSF2 P95R mutations. SRSF2 P95H mutations are associated with older age, increased white blood cell count, and higher VAF values. Co-mutation gene analysis showed that the most common concomitant gene mutation in SRSF2-mutated AML patients was the RUNX1 mutation(33.0%). Survival analysis showed that the 2-year overall survival (OS) and 2-year disease-free survival (DFS) of 57 cases SRSF2-mutated AML patients were 29.6% and 24.7%, respectively. The 2-year OS and DFS were 46.9% and 41.0% in the allo-HSCT group and 20.4% and 11.7% in the chemotherapy group, respectively. The two groups had significant differences in OS(P=0.012 4) and DFS(P=0.013 3). The results of multivariate analysis showed that age was an independent risk factor for OS(HR=2.385, 95%CI 1.074-5.298, P=0.033) and DFS(HR=3.378, 95%CI 1.223-9.334, P=0.019) in SRSF2-mutated AML patients.Conclusion Allo-HSCT can improve the prognosis of AML patients with SRSF2 gene mutation. In addition, age can be an independent risk factor for OS and DFS in AML patients with SRSF2 mutations.-
Key words:
- acute myeloid leukemia /
- gene /
- SRSF2 /
- hematopoietic stem cell transplantation /
- survival analysis
-

-
表 1 57例SRSF2基因突变AML患者的临床特征
临床特征 数值 年龄/岁 60(17~83) >65岁/例(%) 20(35.1) 性别/例(%) 男 42(73.7) 女 15(26.3) AML类型/例(%) De novo AML 43(75.4) sAML 14(24.6) 白细胞计数/(×10[9]/L) 9.91(0.65~149.30) 血红蛋白/(g/L) 71(9~117) 血小板计数/(×10[9]/L) 35(4~566) 骨髓原始细胞/% 43.0(20.0~91.0) 染色体核型/例(%) 正常 26(45.6) <3种异常 16(28.1) 复杂核型 8(14.0) 未知 7(12.3) 伴随基因突变数 4(0~10) SRSF2 VAF/% 39.0(3.0~55.6) 2017版ELN分层/例(%) 预后良好 8(14.0) 预后中等 16(28.1) 预后不良 33(57.9) 接受allo-HSCT 20(35.1) 表 2 SRSF2 P95H基因突变和其他突变类型AML患者临床特征
临床特征 P95H(18例) 其他(39例) P 年龄/岁 63(42~81) 58(17~83) 0.042 性别/例(%) 0.521 男 12(66.7) 30(76.9) 女 6(33.3) 9(23.1) AML类型/例(%) 0.475 De novo AML 12(66.7) 31(79.5) sAML 6(33.3) 8(20.5) 白细胞计数/(×10[9]/L) 17.98(0.75~111.70) 7.18(0.65~149.30) 0.020 血红蛋白/(g/L) 79(9~117) 70(41~110) 0.263 血小板计数/(×10[9]/L) 51.5(16.0~566.0) 34.0(4.0~235.0) 0.419 骨髓原始细胞/% 40.5(20.0~91.0) 44.0(20.0~85.5) 0.918 染色体核型 正常 10(55.6) 16(41.0) 0.174 <3种异常 3(16.7) 13(33.3) 0.193 复杂核型 2(11.1) 6(15.4) 0.983 未知 3(16.7) 4(10.3) 0.667 伴随基因突变数/个 5(2~8) 4(0~10) 0.064 SRSF2 VAF/% 47.0(7.0~53.8) 33.4(3.0~55.6) 0.007 2017版ELN分层 0.005 预后良好 0 8(20.5) 预后中等 2(11.1) 14(35.9) 预后不良 16(88.9) 17(43.6) 接受allo-HSCT/例(%) 5(27.8) 15(38.5) 0.432 表 3 影响SRSF2突变的AML患者OS和DFS的单因素分析
临床特征 OS DFS HR(95%CI) P HR(95%CI) P 年龄(≥65岁vs<65岁) 2.788(1.302~5.971) <0.001 3.717(1.315~10.51) <0.001 性别(男性vs女性) 1.474(0.721~3.014) 0.321 1.412(0.614~3.250) 0.437 复杂核型(是vs否) 0.590(0.226~1.543) 0.365 0.918(0.328~2.574) 0.870 白细胞计数(≥30×10[9]/L vs<30×10[9]/L) 0.820(0.360~1.866) 0.649 0.627(0.251~1.565) 0.364 P95H位点突变(是vs否) 1.116(0.550~2.262) 0.749 1.770(0.764~4.102) 0.122 初诊合并RUNX1(是vs否) 1.496(0.733~3.055) 0.221 1.579(0.676~3.688) 0.227 接受allo-HSCT(是vs否) 0.405(0.210~0.781) 0.012 0.395(0.183~0.852) 0.011 表 4 影响SRSF2突变的AML患者OS和DFS的多因素分析
临床特征 OS DFS HR(95%CI) P HR(95%CI) P 年龄(≥65岁vs<65岁) 2.385(1.074~5.298) 0.033 3.378(1.223~9.334) 0.019 接受allo-HSCT(是vs否) 0.583(0.243~1.399) 0.227 0.634(0.239~1.685) 0.361 -
[1] Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission[J]. Blood, 2016, 127(1): 62-70. doi: 10.1182/blood-2015-07-604546
[2] Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel[J]. Blood, 2017, 129(4): 424-447. doi: 10.1182/blood-2016-08-733196
[3] 张钰, 邵若洋, 刘启发. FLT3突变急性髓系白血病的全程管理[J]. 临床血液学杂志, 2023, 36(5): 303-308. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2023.05.002
[4] Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia[J]. N Engl J Med, 2016, 374(23): 2209-2221. doi: 10.1056/NEJMoa1516192
[5] Jia W, Guo X, Wei Y, et al. Clinical and prognostic profile of SRSF2 and related spliceosome mutations in patients with acute myeloid leukemia[J]. Mol Biol Rep, 2023, 50(8): 6601-6610. doi: 10.1007/s11033-023-08597-w
[6] Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression[J]. Biochem J, 2009, 417(1): 15-27. doi: 10.1042/BJ20081501
[7] Saez B, Walter MJ, Graubert TA. Splicing factor gene mutations in hematologic malignancies[J]. Blood, 2017, 129(10): 1260-1269. doi: 10.1182/blood-2016-10-692400
[8] 中华医学会血液学分会白血病淋巴瘤学组. 成人急性髓系白血病(非急性早幼粒细胞白血病)中国诊疗指南(2017年版)[J]. 中华血液学杂志, 2017, 38(3): 177-182.
[9] Tacke R, Manley JL. The human splicing factors ASF/SF2 and SC35 possess distinct, functionally significant RNA binding specificities[J]. EMBO J, 1995, 14(14): 3540-3551. doi: 10.1002/j.1460-2075.1995.tb07360.x
[10] Kim E, Ilagan JO, Liang Y, et al. SRSF2 Mutations Contribute to Myelodysplasia by Mutant-Specific Effects on Exon Recognition[J]. Cancer Cell, 2015, 27(5): 617-630. doi: 10.1016/j.ccell.2015.04.006
[11] Zhang J, Lieu YK, Ali AM, et al. Disease-associated mutation in SRSF2 misregulates splicing by altering RNA-binding affinities[J]. Proc Natl Acad Sci U S A, 2015, 112(34): E4726-4734.
[12] Palomo L, Garcia O, Arnan M, et al. Targeted deep sequencing improves outcome stratification in chronic myelomonocytic leukemia with low risk cytogenetic features[J]. Oncotarget, 2016, 7(35): 57021-57035. doi: 10.18632/oncotarget.10937
[13] Yoshida K, Sanada M, Shiraishi Y, et al. Frequent pathway mutations of splicing machinery in myelodysplasia[J]. Nature, 2011, 478(7367): 64-69. doi: 10.1038/nature10496
[14] Zhang SJ, Rampal R, Manshouri T, et al. Genetic analysis of patients with leukemic transformation of myeloproliferative neoplasms shows recurrent SRSF2 mutations that are associated with adverse outcome[J]. Blood, 2012, 119(19): 4480-4485. doi: 10.1182/blood-2011-11-390252
[15] Lindsley RC, Mar BG, Mazzola E, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations[J]. Blood, 2015, 125(9): 1367-1376. doi: 10.1182/blood-2014-11-610543
[16] Grimm J, Jentzsch M, Bill M, et al. Clinical implications of SRSF2 mutations in AML patients undergoing allogeneic stem cell transplantation[J]. Am J Hematol, 2021, 96(10): 1287-1294. doi: 10.1002/ajh.26298
[17] Lachowiez CA, Loghavi S, Furudate K, et al. Impact of splicing mutations in acute myeloid leukemia treated with hypomethylating agents combined with venetoclax[J]. Blood Adv, 2021, 5(8): 2173-2183. doi: 10.1182/bloodadvances.2020004173
[18] van der Werf I, Wojtuszkiewicz A, Meggendorfer M, et al. Splicing factor gene mutations in acute myeloid leukemia offer additive value if incorporated in current risk classification[J]. Blood Adv, 2021, 5(17): 3254-3265. doi: 10.1182/bloodadvances.2021004556
[19] Kon A, Yamazaki S, Nannya Y, et al. Physiological Srsf2 P95H expression causes impaired hematopoietic stem cell functions and aberrant RNA splicing in mice[J]. Blood, 2018, 131(6): 621-635. doi: 10.1182/blood-2017-01-762393
[20] Lee SC, Dvinge H, Kim E, et al. Modulation of splicing catalysis for therapeutic targeting of leukemia with mutations in genes encoding spliceosomal proteins[J]. Nat Med, 2016, 22(6): 672-678. doi: 10.1038/nm.4097
[21] Richardson DR, Swoboda DM, Moore DT, et al. Genomic characteristics and prognostic significance of co-mutated ASXL1/SRSF2 acute myeloid leukemia[J]. Am J Hematol, 2021, 96(4): 462-470. doi: 10.1002/ajh.26110
[22] Hou HA, Liu CY, Kuo YY, et al. Splicing factor mutations predict poor prognosis in patients with de novo acute myeloid leukemia[J]. Oncotarget, 2016, 7(8): 9084-9101. doi: 10.18632/oncotarget.7000
-




 下载:
下载: