Efficacy and safety analysis of ixazomib regimen in the treatment of patients with relapsed refractory multiple myeloma
-
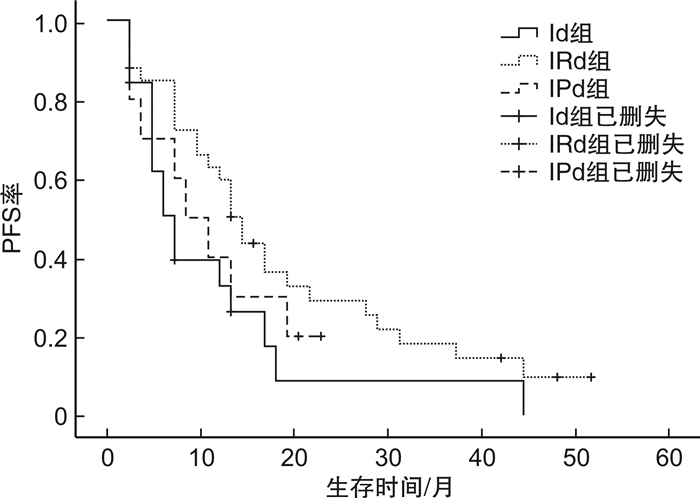
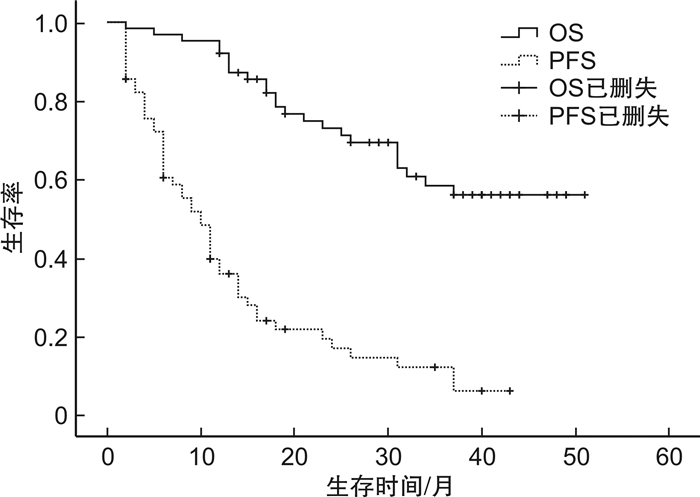
摘要: 目的 观察含伊沙佐米的联合方案治疗复发难治性多发性骨髓瘤(RRMM)的临床疗效及安全性。方法 回顾性分析2020年3月5日—2023年9月1日在徐州医科大学附属医院接受至少2个周期含伊沙佐米方案治疗的62例RRMM患者。对62例患者的临床疗效、不良反应和预后进行分析。结果 所有患者均接受至少2个周期含伊沙佐米方案的治疗,总体反应率为67.7%,完全缓解/严格意义的完全缓解率为11.3%,非常好的部分缓解率为6.5%,部分缓解率为50.0%。中位随访28个月,患者中位无进展生存期为10个月,中位获得反应时间为1.1个月,中位OS未达到。影响患者PFS分析结果显示,年龄≥60岁、细胞遗传学高危是RRMM患者发生进展的独立危险因素。而年龄≥60岁、ISS Ⅲ期以及肾功能不全是影响RRMM患者OS的独立危险因素。血液学不良反应主要为中性粒细胞减少和血小板减少,非血液学不良反应主要是腹泻、感染。Ⅲ~Ⅳ级不良反应事件发生率为22.6%,未见Ⅲ~Ⅳ级周围神经病变。结论 伊沙佐米在RRMM患者中具有良好的疗效,且安全性较高。
-
关键词:
- 伊沙佐米 /
- 多发性骨髓瘤 /
- 复发难治性多发性骨髓瘤 /
- 疗效 /
- 安全性
Abstract: Objective To observe the clinical efficacy and safety of combination regimen containing ixazomib in the treatment of relapsed refractory multiple myeloma(RRMM).Methods A retrospective analysis was performed on 62 patients with RRMM who were treated with at least 2 cycles of treatment with ixazomib regimes in the Affiliated Hospital of Xuzhou Medical University from March 5, 2020 to September 1, 2023. The clinical efficacy, adverse reactions and prognosis of 62 patients were analyzed.Results All patients received at least two cycles of treatment with ixazomib. Overall response rate was 67.7%, complete remission/stringent complete response rate was 11.3%, very good partial remission rate was 6.5%, partial remission rate was 50.0%. At a median follow-up of 28 months, patients had a median progression-free survival of 10 months, a median acquired response time of 1.1 months, and a median overall survival was not achieved. Prognostic analysis showed that age ≥60 years and cytogenetic high risk were independent risk factors for progression in patients with RRMM. Age ≥60 years, ISS stage Ⅲ, and renal insufficiency were all independent risk factors for overall survival in RRMM patients. The main hematological adverse reactions were neutropenia and thrombocytopenia, while the main non-hematological adverse reactions were diarrhea and infection. The incidence of grade Ⅲ-Ⅳ adverse events was 22.6%, and no grade Ⅲ-Ⅳ peripheral neuropathy was observed.Conclusion Ixazomib has good efficacy and safety in patients with relapsed refractory multiple myeloma.-
Key words:
- ixazomib /
- multiple myeloma /
- relapse/refractory multiple myeloma /
- efficacy /
- safety
-

-
表 1 62例RRMM患者的临床特征
基本资料 例数(%) 基本资料 例数(%) 年龄/岁 糖尿病 <60 35(56.5) 是 7(11.3) ≥60 27(43.5) 否 55(88.7) 性别 髓外病灶 男 34(54.8) 伴有 14(22.6) 女 28(45.2) 不伴有 48(77.4) 分型 既往治疗线数 IgA 14(22.6) 1线 17(27.4) IgG 33(53.2) 2线 19(30.6) IgD 1(1.6) ≥3线 26(41.9) 轻链型 12(19.4) 既往治疗情况 寡分泌型 2(3.2) 硼替佐米耐药 ISS分期 是 36(58.1) Ⅰ期 13(21.0) 否 26(41.9) Ⅱ期 24(38.7) 来那度胺耐药 Ⅲ期 25(40.3) 是 45(72.6) DS分期 否 17(27.4) Ⅰ期 34(54.8) ASCT Ⅱ期 7(11.3) 是 27(43.5) Ⅲ期 21(33.9) 否 35(56.5) 细胞遗传学风险分层 CAR-T 是 2(3.2) 标危 39(62.9) 否 60(96.8) 高危 23(37.1) 治疗方案 肾功能 Id 19(30.7) 肾功能正常 48(77.4) IRd 33(53.2) 肾功能不全 14(22.6) IPd 10(16.1) 表 2 不同治疗方案组的疗效
例(%) 疗效 IRd方案组
(n=33)其他方案组 Id方案组
(n=19)IPd方案组
(n=10)ORR 24(72.7) 11(57.9) 7(70.0) CR 4(12.1) 2(10.5) 1(10.0) VGPR 3(9.1) 1(5.3) 0 PR 17(51.5) 8(42.1) 6(60.0) SD 4(12.1) 4(21.1) 1(10.0) PD 5(15.2) 4(21.1) 2(20.0) 表 3 含伊沙佐米方案治疗有效的42例RRMM患者的临床特征与疗效的相关性分析
例(%) 临床特征 有效 P 临床特征 有效 P 临床特征 有效 P 年龄/岁 0.697 分型 0.265 伴有髓外病灶 0.509 <60 19(54.3) IgA 11(78.6) 是 11(78.6) ≥60 23(85.2) IgG 23(69.7) 否 31(64.6) ISS分期 0.094 IgD 0 硼替佐米耐药 0.001 Ⅰ期 8(61.5) 轻链型 6(50.0) 是 18(50.0) Ⅱ期 20(83.3) 寡分泌型 2(100.0) 否 24(92.3) Ⅲ期 14(56.0) 细胞遗传学风险分层 0.002 来那度胺耐药 0.227 DS分期 0.856 标危 32(82.1) 是 28(62.2) Ⅰ期 24(70.6) 高危 10(43.5) 否 14(82.4) Ⅱ期 5(71.4) 肾功能 0.010 既往ASCT 0.697 Ⅲ期 13(61.9) 肾功能正常 37(77.1) 是 19(70.4) 既往治疗线数 0.743 肾功能不全 5(35.7) 否 23(65.7) 1线 11(64.7) 糖尿病 0.823 既往CAR-T 0.207 2线 12(63.2) 是 5(71.4) 是 2(100.0) ≥3线 19(73.1) 否 37(67.3) 否 40(66.7) 表 4 影响62例RRMM患者PFS的单因素分析
影响因素 χ2 P 95%CI 年龄/岁 <60 3.975 0.046 5.170~10.830 ≥60 7.477~14.523 ISS分期 Ⅰ~Ⅱ期 1.715 0.190 9.031~12.969 Ⅲ期 3.269~8.731 免疫分型 其他分型 <0.001 0.999 4.728~11.272 IgG 8.263~13.737 高危细胞遗传学 正常 10.750 0.001 9.385~14.615 异常 3.505~6.495 肾功能 肾功能正常 6.031 0.014 8.561~13.439 肾功能不全 4.829~7.171 伴有髓外病灶 否 4.650 0.031 7.368~14.632 是 0.500~11.500 既往治疗线数/线 1~2 0.549 0.459 4.398~7.602 ≥3 9.797~12.203 硼替佐米耐药 否 0.001 0.971 7.628~12.372 是 4.994~17.006 既往ASCT 否 0.898 0.343 4.667~11.333 是 8.567~13.433 既往CAR-T 否 0.952 0.329 8.618~13.382 是 — 表 5 影响62例RRMM患者PFS的多因素分析
影响因素 β SE Wald χ2 P HR 95%CI 年龄 0.623 0.298 4.361 0.037 0.536 0.299~0.962 高危细胞遗传学 0.878 0.343 6.558 0.010 2.407 1.229~4.715 肾功能 0.542 0.385 1.982 0.159 1.720 0.808~3.660 伴有髓外病灶 0.562 0.353 2.534 0.111 1.754 0.878~3.502 表 6 影响62例RRMM患者OS的单因素分析
影响因素 χ2 P 95%CI 年龄/岁 <60 4.822 0.028 35.291~44.668 ≥60 16.162~45.838 ISS分期 Ⅰ~Ⅱ期 12.360 0.002 31.684~40.546 Ⅲ期 17.880~24.120 免疫分型 其他分型 0.191 0.662 29.515~38.664 IgG 28.713~40.020 血钙/(mmol/L) <2.6 11.329 <0.001 34.517~41.371 ≥2.6 19.065~24.935 Hb/(g/L) >100 6.730 0.009 35.292~44.300 ≤100 14.432~35.568 高危细胞遗传学 正常 4.483 0.034 33.812~42.704 异常 16.922~25.078 肾功能 肾功能正常 6.067 0.014 33.160~41.061 肾功能不全 15.558~24.192 伴有髓外病灶 否 0.410 0.522 31.410~40.341 是 26.478~41.522 既往治疗线数/线 1~2 0.036 0.849 30.014~41.276 ≥3 26.866~35.263 硼替佐米耐药 否 0.021 0.885 29.207~40.805 是 28.642~37.817 既往ASCT 否 0.433 0.511 31.169~42.012 是 27.261~36.965 既往CAR-T 否 0.892 0.345 — 是 — 表 7 影响62例RRMM患者OS的多因素分析
影响因素 β SE Wald χ2 P HR 95%CI 年龄 1.433 0.513 7.820 0.005 4.192 1.535~11.447 ISS分期 1.230 0.484 6.459 0.011 3.422 1.325~8.836 血钙值 0.745 0.531 1.969 0.161 2.106 0.744~5.962 Hb值 0.633 0.455 1.936 0.164 1.883 0.772~4.594 高危细胞遗传学 0.874 0.449 3.788 0.052 2.397 0.994~5.783 肾功能 1.249 0.601 4.328 0.037 3.488 1.075~11.317 表 8 RRMM患者伊沙佐米治疗期间不良反应发生率比较
例(%) 不良反应 所有等级 Ⅰ~Ⅱ级 Ⅲ~Ⅳ级 血液学 中性粒细胞减少症 11(17.7) 9(14.5) 2(3.2) 血小板减少症 10(16.1) 9(14.5) 1(1.6) 贫血 7(11.3) 4(6.5) 3(4.8) 非血液学 周围神经病变 6(9.7) 6(9.7) 0 皮疹 11(17.7) 10(16.1) 1(1.6) 带状疱疹 4(6.5) 4(6.5) 0 恶心 7(11.3) 7(11.3) 0 呕吐 6(9.7) 6(9.7) 0 腹痛 6(9.7) 6(9.7) 0 腹泻 14(22.6) 11(17.7) 3(4.8) 感染 11(17.7) 10(16.1) 1(1.6) 实验室检验 肌酸酐增加 4(6.5) 3(4.8) 1(1.6) ALT/AST比值升高 2(3.2) 2(3.2) 0 低钾血症 8(12.9) 5(8.1) 3(4.8) 心血管系统 1(1.6) 1(1.6) 0 -
[1] Facon T, Leleu X, Manier S. How I treat multiple myeloma in geriatric patients[J]. Blood, 2024, 143(3): 224-232. doi: 10.1182/blood.2022017635
[2] Morè S, Corvatta L, Manieri VM, et al. Current main topics in multiple myeloma[J]. Cancers, 2023, 15(8): 2203. doi: 10.3390/cancers15082203
[3] Sahu I, Glickman MH. Proteasome in action: substrate degradation by the 26S proteasome[J]. Biochem Soc Trans, 2021, 49(2): 629-644. doi: 10.1042/BST20200382
[4] Spano D, Catara G. Targeting the ubiquitin-proteasome system and recent advances in cancer therapy[J]. Cells, 2023, 13(1): 29. doi: 10.3390/cells13010029
[5] 徐燕, 邱录贵. 蛋白酶体抑制剂在多发性骨髓瘤治疗中的应用[J]. 中国肿瘤临床, 2022, 49(20): 1028-1032.
[6] Diaz-DelCastillo M, Gundesen M, Andersen C, et al. Ixazomib-a new generation proteasome inhibitor-promotes bone formation in multiple myeloma[J]. J Bone Mineral Res, 2023, 38: 207-207.
[7] Richardson PG, Kumar SK, Masszi T, et al. Final overall survival analysis of the TOURMALINE-MM1 phase Ⅲ trial of ixazomib, lenalidomide, and dexamethasone in patients with relapsed or refractory multiple myeloma[J]. J Clin Oncol, 2021, 39(22): 2430-2442. doi: 10.1200/JCO.21.00972
[8] Moreau P, Kumar SK, San Miguel J, et al. Treatment of relapsed and refractory multiple myeloma: recommendations from the international myeloma working group[J]. Lancet Oncol, 2021, 22(3): e105-e118. doi: 10.1016/S1470-2045(20)30756-7
[9] 李莹, 王耀美, 崔玉山, 等. 塞利尼索为基础方案治疗10例复发难治多发性骨髓瘤疗效观察[J]. 临床血液学杂志, 2023, 36(5): 327-331. doi: 10.13201/j.issn.1004-2806.2023.05.006
[10] 李芸, 李兵胜, 李义秀, 等. 伊沙佐米治疗复发/难治性多发性骨髓瘤的疗效和安全性的系统评价[J]. 中国医院用药评价与分析, 2024, 24(4): 473-477.
[11] 陈冉, 薛连国, 周航, 等. 含伊沙佐米的联合方案治疗多发性骨髓瘤患者的临床疗效及安全性分析[J]. 中国实验血液学杂志, 2024, 32(2): 483-492.
[12] 向倩, 任诗慧, 李晓明. 伊沙佐米治疗多发性骨髓瘤的疗效性与安全性的Meta分析[J]. 临床输血与检验, 2023, 25(2): 243-249.
[13] Terpos E, Ntanasis-Stathopoulos I, Gavriatopoulou M, et al. Efficacy and safety of daratumumab with ixazomib and dexamethasone in lenalidomide-exposed patients after one prior line of therapy: final results of the phase 2 study DARIA[J]. Am J Hematol, 2024, 99(3): 396-407.
[14] Facon T, Venner CP, Bahlis NJ, et al. Oral ixazomib, lenalidomide, and dexamethasone for transplant-ineligible patients with newly diagnosed multiple myeloma[J]. Blood, 2021, 137(26): 3616-3628.
[15] Fu CC, Zhou X, Zhuang JL, et al. Characteristics and safety profile in patient received ixazomib in China: interim analysis of a national, prospective, non-interventional study of using ixazomib in real-world clinical practice(INFINITE study)[J]. Blood, 2022, 140(Supplement 1): 12642-12644.
[16] 王婧, 顾伟英, 管俊, 等. 真实世界伊沙佐米治疗多发性骨髓瘤疗效与安全性研究[J]. 中国实用内科杂志, 2023, 43(1): 52-57, 68.
[17] Macro M, Hulin C, Vincent L, et al. Real-world effectiveness of ixazomib combined with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: the REMIX study[J]. Ann Hematol, 2023, 102(8): 2137-2151.
[18] Ding K, Yu H, Shao YY, et al. Real-world data on the efficacy and safety of ixazomib-based therapy in multiple myeloma: a single-center study in China[J]. Cancer Manag Res, 2020, 12: 8935-8941.
[19] Liu AJ, Yu H, Hou R, et al. Assessment of prolonged proteasome inhibition through ixazomib-based oral regimen on newly diagnosed and first-relapsed multiple myeloma: a real-world Chinese cohort study[J]. Cancer Med, 2024, 13(9): e7177.
[20] 桂琳, 陈宝安, 高冲, 等. 多发性骨髓瘤患者总生存期的非遗传学影响因素分析[J]. 中国实验血液学杂志, 2020, 28(6): 1946-1951.
[21] Solmaz S, Uzun O, Sevindik OG, et al. The effect of haemoglobin, albumin, lymphocyte and platelet score on the prognosis in patients with multiple myeloma[J]. Int J Lab Hematol, 2023, 45(1): 13-19.
[22] Ludwig H, Ramasamy K, Mateos MV, et al. Use via early access to ixazomib(UVEA-IXA)study: effectiveness and safety of ixazomib-based therapy in relapsed/refractory multiple myeloma outside of the clinical trial setting[J]. Clin Lymphoma Myeloma Leuk, 2024, 24(2): e40-e49. e3.
[23] Yang Y, Zhao B, Lan HL, et al. Bortezomib-induced peripheral neuropathy: clinical features, molecular basis, and therapeutic approach[J]. Crit Rev Oncol, 2024, 197: 104353.
[24] 王科, 苏梅芳, 王萌, 等. 硼替佐米不同给药途径治疗多发性骨髓瘤的疗效及其与周围神经病变的相关分析[J]. 临床内科杂志, 2022, 39(12): 846-847.
[25] 左晓佳, 叶丽霖, 冯尽意, 等. 硼替佐米不同给药途径对多发性骨髓瘤周围神经病变的影响[J]. 实用药物与临床, 2021, 24(6): 498-500.
-

计量
- 文章访问数: 175
- 施引文献: 0



 下载:
下载: