Comparison in the predictive performance of different endothelial activation and stress index scoring systems for coagulation dysfunction after CAR-T therapy in patients with multiple myeloma
-
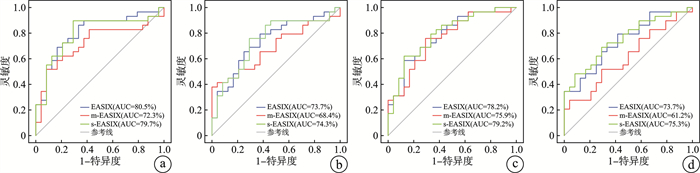
摘要: 目的 比较不同内皮活化和应激指数(EASIX)评分预测多发性骨髓瘤患者嵌合抗原受体T细胞(chimeric antigen receptor T cell,CAR-T)治疗后出现凝血功能障碍的效能。方法 选取2019年5月—2022年10月于徐州医科大学附属医院血液科住院治疗行CAR-T细胞输注、且满足入组条件的53例多发性骨髓瘤患者,收集临床资料进行回顾性分析。构建受试者工作特征(ROC)曲线,计算曲线下面积,并进行比较。采用单因素logistic回归分析EASIX评分(乳酸脱氢酶×肌酐/血小板)及2个改良EASIX评分[简化EASIX(s-EASIX)即不包括肌酐的EASIX;改良EASIX(m-EASIX)即用C反应蛋白代替肌酐的EASIX]在不同时间点与凝血功能障碍之间的关系。结果 53例患者中,男35例(66%),女18例(34%),中位年龄为57(31~70)岁,29例(54.7%)患者发生了凝血功能障碍。根据EASIX、s-EASIX、m-EASIX评分在不同时间点ROC曲线下面积(area under curve,AUC)的结果表明,在淋巴细胞清除预处理前,EASIX的AUC值最大(80.5%),在第0天、第7天及第14天,s-EASIX的AUC值最大(74.3% vs 79.2% vs 75.3%,P < 0.05)。单因素分析显示CAR-T细胞治疗后凝血功能障碍的发生与血小板的减少显著相关(P < 0.05)。结论 s-EASIX评分是预测多发性骨髓瘤患者CAR-T细胞治疗后出现凝血功能障碍的最佳评分系统,其次是EASIX评分。Abstract: Objective To compare the efficacy of different endothelial activation and stress index(EASIX) scores in predicting coagulation dysfunction after chimeric antigen receptor T cell(CAR-T) therapy in patients with multiple myeloma.Methods A total of 53 patients with multiple myeloma who underwent CAR-T cell transfusion in the department of hematology, affiliated hospital of Xuzhou Medical University from May 2019 to October 2022 and met the enrollment conditions were selected and the clinical data were collected for retrospective analysis. The receiver operating characteristic curve was constructed, the area under the curve was calculated and compared with each other. The major logistic regression analysis was used to analyze the relationship between coagulation dysfunction and scoring systems of EASIX (lactate dehydrogenase×creatinine / platelet) and the two modified EASIX(simplified EASIX[s-EASIX]was EASIX excluding creatinine; modified EASIX[m-EASIX]was EASIX substituting CRP for creatinine) at different time points.Results Among the 53 patients, 35(66%) were males and 18(34%) were females, with a median age of 57(31-70) years, and coagulation disorders occurred in 29(54.7%) patients. The results based on the area under curve(AUC) of EASIX, s-EASIX, and m-EASIX scores at different time points of the ROC curve showed that the AUC value of EASIX was the highest(80.5%) before lymphocyte clearance pretreatment, and the AUC value of s-EASIX was the highest on days 0, 7, and 14(74.3% vs 79.2% vs 75.3%, P < 0.05). The univariate analysis showed that the occurrence of congratulations dysfunction was significantly associated with the decrease in platelets after CAR-T cell therapy(P < 0.05).Conclusion The s-EASIX score is the best scoring system for predicting coagulation dysfunction after CAR-T cell therapy in patients with multiple myeloma, followed by the EASIX score.
-
Key words:
- multiple myeloma /
- CAR-T cells /
- EASIX score /
- modified EASIX score /
- coagulation dysfunction /
- predictive performance
-

-
表 1 所有CAR-T治疗患者的基线特征例(%),中位数(范围)
变量 总计(n=53) 性别 女 18(34.0) 男 35(66.0) 年龄/岁 57(31~70) ISS分期 Ⅰ期 9(17.0) Ⅱ期 26(49.1) Ⅲ期 18(34.0) 既往治疗线/线 4(2~8) 自体造血干细胞移植 无 40(75.5) 有 13(24.5) CRS分级 1~2级 42(79.2) 3~4级 5(9.4) ICANS分级 1~2级 1(1.9) 3~4级 1(1.9) 凝血功能障碍 无 24(45.3) 有 29(54.7) 表 2 EASIX、m-EASIX、s-EASIX评分在各个时间点ROC曲线参数值
评分 AUC/% 灵敏度 特异度 约登指数 最佳截断值 P 淋巴细胞清除预处理前 EASIX 80.5 0.862 0.667 0.529 1.41 < 0.01 m-EASIX 72.3 0.517 0.917 0.434 82.99 < 0.01 s-EASIX 79.7 0.897 0.708 0.605 1.98 < 0.01 第0天 EASIX 73.7 0.793 0.625 0.418 1.51 < 0.01 m-EASIX 68.4 0.379 1.000 0.379 198.81 0.022 s-EASIX 74.3 0.759 0.708 0.467 2.46 < 0.01 第7天 EASIX1 78.2 0.586 0.875 0.461 4.91 < 0.01 m-EASIX1 75.9 0.759 0.708 0.467 45.12 < 0.01 s-EASIX1 79.2 0.621 0.875 0.496 6.16 < 0.01 第14天 EASIX 73.7 0.793 0.583 0.376 3.55 < 0.01 m-EASIX 61.2 0.276 0.958 0.234 1 401.63 0.163 s-EASIX 75.3 0.483 0.917 0.399 26.77 < 0.01 表 3 各个评分中包含的单个变量与凝血功能障碍的关系
变量 例数 OR(95%CI) P 淋巴细胞清除预处理前 LDH 53 1.003(0.999~1.007) 0.099 肌酐 53 21.457(0.285~1 618.127) 0.159 CRP 53 1.002(0.981~1.024) 0.850 PLT 53 0.984(0.975~0.994) < 0.010 第0天 LDH 53 1.002(0.998~1.006) 0.237 肌酐 53 4.892(0.069~348.937) 0.466 CRP 53 1.012(0.993~1.031) 0.226 PLT 53 0.987(0.977~0.996) < 0.010 第7天 LDH 53 1.001(0.999~1.004) 0.230 肌酐 53 1.908(0.314~11.588) 0.483 CRP 53 1.011(0.995~1.027) 0.182 PLT 53 0.985(0.975~0.995) < 0.010 第14天 LDH 53 1.003(1.001~1.005) 0.016 肌酐 53 1.836(0.183~18.438) 0.606 CRP 53 1.008(0.995~1.021) 0.215 PLT 53 0.988(0.977~0.998) 0.019 -
[1] Zhang X, Zhang H, Lan H, et al. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies[J]. Front Immunol, 2023, 14: 1101495. doi: 10.3389/fimmu.2023.1101495
[2] Mei H, Chen F, Han Y, et al. Chinese expert consensus on the management of chimeric antigen receptor T cell therapy-associated coagulopathy[J]. Chin Med J(Engl), 2022, 135(14): 1639-1641.
[3] Ishihara T, Arai Y, Morita M, et al. Suppressed Fibrinolytic Activity Demonstrated By Simultaneous Thrombin and Plasmin Generation Assay during Cytokine Release Syndrome after CD19 Chimeric Antigen Receptor-Modified T-Cell Therapy[J]. Blood, 2021, 138(Supplement 1): 4807. doi: 10.1182/blood-2021-146311
[4] Luft T, Benner A, Jodele S, et al. EASIX in patients with acute graft-versus-host disease: a retrospective cohort analysis[J]. Lancet Haematol, 2017, 4(9): e414-e423. doi: 10.1016/S2352-3026(17)30108-4
[5] Krüger-Genge A, Blocki A, Franke RP, et al. Vascular Endothelial Cell Biology: An Update[J]. Int J Mol Sci, 2019, 20(18): 4411. doi: 10.3390/ijms20184411
[6] Zhao Y, Zhang X, Zhang M, et al. Modified EASIX scores predict severe CRS/ICANS in patients with acute myeloid leukemia following CLL1 CAR-T cell therapy[J]. Ann Hematol, 2024, 103(3): 969-980. doi: 10.1007/s00277-024-05617-y
[7] Pennisi M, Sanchez-Escamilla M, Flynn JR, et al. Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells[J]. Blood Adv, 2021, 5(17): 3397-3406. doi: 10.1182/bloodadvances.2020003885
[8] Song GY, Jung SH, Kim K, et al. Endothelial activation and stress index(EASIX)is a reliable predictor for overall survival in patients with multiple myeloma[J]. BMC Cancer, 2020, 20(1): 803. doi: 10.1186/s12885-020-07317-y
[9] Wang Y, Qi K, Cheng H, et al. Coagulation Disorders after Chimeric Antigen Receptor T Cell Therapy: Analysis of 100 Patients with Relapsed and Refractory Hematologic Malignancies[J]. Biol Blood Marrow Transplant, 2020, 26(5): 865-875. doi: 10.1016/j.bbmt.2019.11.027
[10] Buechner J, Grupp SA, Hiramatsu H, et al. Practical guidelines for monitoring and management of coagulopathy following tisagenlecleucel CAR T-cell therapy[J]. Blood Adv, 2021, 5(2): 593-601. doi: 10.1182/bloodadvances.2020002757
[11] Santomasso BD, Nastoupil LJ, Adkins S, et al. Management of Immune-Related Adverse Events in Patients Treated With Chimeric Antigen Receptor T-Cell Therapy: ASCO Guideline[J]. J Clin Oncol, 2021, 39(35): 3978-3992. doi: 10.1200/JCO.21.01992
[12] Bruno B, Wäsch R, Engelhardt M, et al. European Myeloma Network perspective on CAR T-Cell therapies for multiple myeloma[J]. Haematologica, 2021, 106(8): 2054-2065. doi: 10.3324/haematol.2020.276402
[13] Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells[J]. Biol Blood Marrow Transplant, 2019, 25(4): 625-638. doi: 10.1016/j.bbmt.2018.12.758
[14] Wang X, Li C, Luo W, et al. IL-10 plus the EASIX score predict bleeding events after anti-CD19 CAR T-cell therapy[J]. Ann Hematol, 2023, 102(12): 3575-3585. doi: 10.1007/s00277-023-05477-y
[15] Jess J, Yates B, Dulau-Florea A, et al. CD22 CAR T-cell associated hematologic toxicities, endothelial activation and relationship to neurotoxicity[J]. J Immunother Cancer, 2023, 11(6): e005898. doi: 10.1136/jitc-2022-005898
[16] Chopra J, Joist JH, Webster RO. Loss of 51 chromium, lactate dehydrogenase, and 111indium as indicators of endothelial cell injury[J]. Lab Invest, 1987, 57(5): 578-584.
[17] Nachman RL, Rafii S. Platelets, petechiae, and preservation of the vascular wall[J]. N Engl J Med, 2008, 359(12): 1261-1270. doi: 10.1056/NEJMra0800887
[18] Cadamuro M, Brivio S, Mertens J, et al. Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma[J]. J Hepatol, 2019, 70(4): 700-709. doi: 10.1016/j.jhep.2018.12.004
[19] Sun Y, Liu XL, Zhang D, et al. Platelet-Derived Exosomes Affect the Proliferation and Migration of Human Umbilical Vein Endothelial Cells Via miR-126[J]. Curr Vasc Pharmacol, 2019, 17(4): 379-387. doi: 10.2174/1570161116666180313142139
[20] Du M, Huang L, Kou H, et al. Case Report: ITP Treatment After CAR-T Cell Therapy in Patients With Multiple Myeloma[J]. Front Immunol, 2022, 13: 898341. doi: 10.3389/fimmu.2022.898341
[21] Wudhikarn K, Pennisi M, Garcia-Recio M, et al. DLBCL patients treated with CD19 CAR T cells experience a high burden of organ toxicities but low nonrelapse mortality[J]. Blood Adv, 2020, 4(13): 3024-3033. doi: 10.1182/bloodadvances.2020001972
[22] Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies[J]. Blood Adv, 2020, 4(15): 3776-3787. doi: 10.1182/bloodadvances.2020002509
[23] 吕雨琦, 张明明, 魏国庆, 等. BCMA靶向的嵌合抗原受体T细胞治疗复发/难治多发性骨髓瘤患者发生急性肾损伤的危险因素[J]. 浙江大学学报(医学版), 2022, 51(2): 137-143.
[24] Greenbaum U, Strati P, Saliba RM, et al. CRP and ferritin in addition to the EASIX score predict CAR-T-related toxicity[J]. Blood Adv, 2021, 5(14): 2799-2806. doi: 10.1182/bloodadvances.2021004575
[25] Nissani A, Lev-Ari S, Meirson T, et al. Comparison of non-myeloablative lymphodepleting preconditioning regimens in patients undergoing adoptive T cell therapy[J]. J Immunother Cancer, 2021, 9(5): e001743. doi: 10.1136/jitc-2020-001743
[26] Ferreyro BL, Scales DC, Wunsch H, et al. Critical illness in patients with hematologic malignancy: a population-based cohort study[J]. Intensive Care Med, 2021, 47(10): 1104-1114. doi: 10.1007/s00134-021-06502-2
-




 下载:
下载: