Clinical and molecular genetic characteristics of B-cell acute lymphoblastic leukemia with PAX5 P80R
-
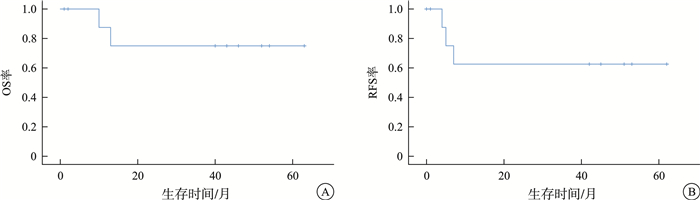
摘要: 目的 分析伴PAX5 P80R突变的急性B淋巴细胞白血病(B-ALL)的临床和分子遗传学特征,探索该亚型患者治疗方案与预后的关系。方法 回顾性研究2019年9月—2024年11月苏州大学附属第一医院和苏州弘慈血液病医院收治的B-ALL患者中,伴PAX5 P80R突变患者的临床资料。结果 936例B-ALL患者中,10例(1.1%)伴PAX5 P80R。其中,男性占80%,初诊中位年龄为35.5(14.0~59.0)岁。10例患者均表达CD10、CD19,其中7例患者还表达CD34和CD38。4例患者合并染色体异常,所有患者融合基因均阴性。7例患者合并22个共突变,其中NRAS最为常见(5/7)。异常核型患者伴有2个以上共突变比例明显高于正常核型患者(100.0% vs 16.6%,P=0.02)。经过基于VP(长春地辛+地塞米松)方案的多药联合诱导化疗后,10例患者均达到形态学完全缓解(CR),其中5例(50%)流式微小残留病(MFC-MRD)阴性(< 0.01%);巩固治疗期间,4例在CR1期间行异基因造血干细胞移植(allo-HSCT)的患者存活,3例患者化疗期间出现复发(2例死亡,1例经移植、嵌合抗原受体T细胞治疗后存活),2例化疗患者无复发生存[1例儿童患者已完成中国儿童白血病协作组-2015化疗方案(CCCG-ALL-2015),1例拟行allo-HSCT],其余1例患者失访。中位随访时间42(2~63)个月,5年总生存率(OS)及无复发生存率(PFS)分别为75%(95%CI 0.153~0.503)和62.5%(95%CI 0.171~0.365),中位OS和PFS均未达到。结论 PAX5 P80R突变的B-ALL对诱导化疗敏感,总体预后良好,但合并其他高危遗传学异常对预后的影响有待进一步探索。Abstract: Objective To analyze the clinical and molecular genetic features of B-cell acute lymphoblastic leukemia(B-ALL) with PAX5 P80R and explore the correlation between treatment approaches and clinical outcomes.Methods A retrospective study was conducted on clinical data of B-ALL patients with PAX5 P80R mutation admitted to the First Affiliated Hospital of Soochow University and Soochow Hopes Hematology Hospital from September 2019 to November 2024.Results In a cohort of 936 patients diagnosed with B-ALL, 10 cases(1.1%) were identified PAX5 P80R mutation. Among these cases, 80% were male, with a median age of 35.5 years at diagnosis(range 14-59 years). All 10 patients expressed CD10 and CD19, with 7 of them also expressing CD34 and CD38. Four patients had chromosomal abnormalities, while all patients tested negative for fusion genes. Seven patients had 22 co-mutations, with NRAS being the most common(5/7). The proportion of patients with more than two co-mutations was significantly higher in those with abnormal karyotypes compared to those with normal karyotypes(100.0% vs 16.6%, P=0.02). Following induction chemotherapy based with VP regimen, all patients achieved complete remission(CR), with a 50% rate of minimal residual disease(MRD) negativity by flow cytometry. During consolidation therapy, 4 patients underwent allogeneic hematopoietic stem cell transplantation(allo-HSCT) in CR1. Of the remaining patients, 3 experienced relapse(2 deaths, 1 survived after allo-HSCT and chimeric antigen receptor T cell therapy), while 2 were still alive without recurrence(1 pediatric patient completed the CCCG-ALL-2015 protocol, and 1 was planning for allo-HSCT). One patient was lost to follow-up. The median follow-up was 42 months(range 2-63 months), with 5-year overall survival(OS) and progression-free survival(PFS) rates of 75%(95%CI 0.153-0.503) and 62.5%(95%CI 0.171-0.365), respectively. The median OS and PFS were not reached.Conclusion B-ALL with PAX5 P80R shows positive responses to induction chemotherapy, with an overall good prognosis. However, the impact of concomitant high-risk genetic abnormalities on prognosis remains to be further explored.
-

-
表 1 10例伴PAX5 P80R阳性B-ALL患者的临床特征
例号 年龄 性别 临床表现 白细胞计数/(×109/L) 骨髓原始细胞比例/% 免疫表型 染色体核型 RT-PCR RNA-seq 伴随突变 1 14 男 胸闷,皮肤瘀点 42.30 94.0 CD34、CD10、CD19 46,XY 阴性 未做 无 2 59 男 乏力 48.54 41.5 CD34、CD10、CD19、CD38、CD22 46,XY 阴性 未做 NRAS 3 18 女 皮肤瘀点 30.68 94.0 CD10、CD19、CD38、CD34 46,XX,t(7;18)(p11;q23)[11]/46,XX[2] 阴性 阴性 NRAS、KMT2D、SOS1 4 43 男 腹痛 3.78 63.0 CD22、CD10、CD19、CD34、 46,XY 阴性 未做 KRAS 5 18 男 头晕乏力 11.21 80.0 CD34、DR、CD10、CD19、CD38 46,XY 阴性 未做 无 6 29 女 皮肤瘀点、瘀斑伴牙龈出血 26.23 87.0 CD34、DR、CD10、CD19、CD38 44,XX,-7,-9,20q-[9]/46,XX[1] 阴性 阴性 DNMT3A、ETV6、NF1、PTPN11、IL7R 7 52 男 体检发现血小板减少 10.73 80.0 CD10、CD19、CD38 45,XY,dic(7;9)(q10;q10)[10] 阴性 未做 NRAS、KRAS、CCND3、CREBBP 8 36 男 头晕,牙龈肿胀 17.70 95.5 CD10、CD19、CD38、CD20 46,XY 阴性 阴性 无 9 35 男 发热 10.21 92.5 CD34、DR、CD10、CD19、CD38 34-46,XY,-1,-2,-4,-5,-6,-8,-9,-10,-12,-12,-14,-15,-17,+18,-19,-20,-22[10] 阴性 未做 NRAS、KMT2D、GNAI2 10 58 男 发热伴乏力 41.00 83.5 CD10、CD19、CD22 46,XY 阴性 阴性 TP53、FLT3、PTPN11、NRAS、KRAS 注:RNA-seq:转录组测序。 表 2 10例伴PAX5 P80R阳性B-ALL患者的治疗、疗效及生存情况
例号 诱导化疗方案/疗效/流式MRD 巩固化疗方案/疗效 复发 再诱导化疗方案/疗效 移植 生存情况
(死亡原因)OS期/月 1 IVLP/CR/ < 3.4×10-4 CCCG-ALL-2015/CR 否 否 否 存活 63.0 2 IVLP/CR/1.20% Hyper-CVAD-B/A/B/CR;VP/复发 是 Hyper-CVAD-B/NR;FC/死亡 否 死亡
(多脏器功能衰竭)10.0 3 IVP/CR/ < 7.5×10-5 Hyper-CVAD-B/MD-MTX/CR 否 否 是(CR1) 存活 54.0 4 IVP /CR/ < 9.4×10-5 MD-MTX/IVP/CR 否 否 是(CR1) 存活 52.0 5 IVP/CR/9.0×10-5 MD-MTX/IVP/CR 否 否 是(CR1) 存活 46.0 6 IVP/CR/ < 3.2×10-4 MD-MTX+PEG-Asp/CR;MD-MTX/CNSL;Hyper-CVAD-A/复发 是 CDA+Ara-C+Vp16+Ven/NR;Blincyto/NR;CD19 CAR-T/NR 否 死亡
(感染)13.0 7 IVP/CR/ < 1.0×10-4 MD-Ara-C+IVP/CR 否 否 是(CR1) 存活 43.0 8 VDP/CR/7×10-5 VP/复发 是 EA/NR;VDCPP/CR2;Hyper-CVAD-B/CR2;移植后60 d复发,FC+ HD-MTX/CR3;AZA/复发;CD19/CD22 CAR-T(供体来源)/CR4 是(CR2) 存活 40.0 9 IVP+Blincyto/CR/6.4×10-4 失访 失访 失访 失访 失访 失访 10 IVP+Blincyto/CR/ < 1.8×10-4 Hyper-CVAD-A/CR 否 否 拟移植 存活 2.0 注:IVLP:伊达比星+长春地辛+培门冬酶+地塞米松;FC:氟达拉滨+环磷酰胺;IVP:伊达比星+长春地辛+地塞米松;CDA:克拉曲滨;Vp16:依托泊苷;Ven:维奈克拉;MD-Ara-C:中剂量阿糖胞苷;VDP:长春地辛+柔红霉素+地塞米松;EA:依托泊苷+阿糖胞苷;VDCPP:长春地辛+柔红霉素+环磷酰胺+培门冬酶+地塞米松;HD-MTX:高剂量甲氨蝶呤;AZA:阿扎胞苷。 -
[1] Hunger SP, Mullighan CG. Redefining ALL classification: toward detecting high-risk ALL and implementing precision medicine[J]. Blood, 2015, 125(26): 3977-3987. doi: 10.1182/blood-2015-02-580043
[2] Lafage-Pochitaloff M, Baranger L, Hunault M, et al. Impact of cytogenetic abnormalities in adults with Ph-negative B-cell precursor acute lymphoblastic leukemia[J]. Blood, 2017, 130(16): 1832-1844. doi: 10.1182/blood-2017-05-783852
[3] Moorman AV, Barretta E, Butler ER, et al. Prognostic impact of chromosomal abnormalities and copy number alterations in adult B-cell precursor acute lymphoblastic leukaemia: a UKALL14 study[J]. Leukemia, 2022, 36(3): 625-636. doi: 10.1038/s41375-021-01448-2
[4] Nebral K, Denk D, Attarbaschi A, et al. Incidence and diversity of PAX5 fusion genes in childhood acute lymphoblastic leukemia[J]. Leukemia, 2009, 23(1): 134-143. doi: 10.1038/leu.2008.306
[5] Cazzaniga G, Daniotti M, Tosi S, et al. The paired box domain gene PAX5 is fused to ETV6/TEL in an acute lymphoblastic leukemia case[J]. Cancer Res, 2001, 61(12): 4666-4670.
[6] Passet M, Boissel N, Sigaux F, et al. PAX5 P80R mutation identifies a novel subtype of B-cell precursor acute lymphoblastic leukemia with favorable outcome[J]. Blood, 2019, 133(3): 280-284. doi: 10.1182/blood-2018-10-882142
[7] 中国抗癌协会血液肿瘤专业委员会, 中华医学会血液学分会白血病淋巴瘤学组. 中国成人急性淋巴细胞白血病诊断与治疗指南(2016年版)[J]. 中华血液学杂志, 2016, 37(10): 837-845.
[8] Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia[J]. Nature, 2007, 446(7137): 758-764. doi: 10.1038/nature05690
[9] Theunissen P, Mejstrikova E, Sedek L, et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia[J]. Blood, 2017, 129(3): 347-357. doi: 10.1182/blood-2016-07-726307
[10] Gu Z, Churchman ML, Roberts KG, et al. PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia[J]. Nat Genet, 2019, 51(2): 296-307. doi: 10.1038/s41588-018-0315-5
[11] Paietta E, Roberts KG, Wang V, et al. Molecular classification improves risk assessment in adult BCR-ABL1-negative B-ALL[J]. Blood, 2021, 138(11): 948-958. doi: 10.1182/blood.2020010144
[12] Jung M, Schieck M, Hofmann W, et al. Frequency and prognostic impact of PAX5 p. P80R in pediatric acute lymphoblastic leukemia patients treated on an AIEOP-BFM acute lymphoblastic leukemia protocol[J]. Genes Chromosomes Cancer, 2020, 59(11): 667-671. doi: 10.1002/gcc.22882
[13] Bastian L, Schroeder MP, Eckert C, et al. PAX5 biallelic genomic alterations define a novel subgroup of B-cell precursor acute lymphoblastic leukemia[J]. Leukemia, 2019, 33(8): 1895-1909. doi: 10.1038/s41375-019-0430-z
[14] Iacobucci I, Mullighan CG. Genetic basis of acute lymphoblastic leukemia[J]. J Clin Oncol, 2017, 35(9): 975-983. doi: 10.1200/JCO.2016.70.7836
[15] Lejman M, Chałupnik A, Chilimoniuk Z, et al. Genetic Biomarkers and Their Clinical Implications in B-Cell Acute Lymphoblastic Leukemia in Children[J]. Int J Mol Sci, 2022, 23(5): 2755. doi: 10.3390/ijms23052755
[16] Lilljebjörn H, Fioretos T. New oncogenic subtypes in pediatric B-cell precursor acute lymphoblastic leukemia[J]. Blood, 2017, 130(12): 1395-1401. doi: 10.1182/blood-2017-05-742643
[17] Roberts KG, Mullighan CG. The biology of B-progenitor acute lymphoblastic leukemia[J]. Cold Spring Harb Perspect Med, 2020, 10(7): a034835. doi: 10.1101/cshperspect.a034835
[18] Pui CH, Nichols KE, Yang JJ. Somatic and germline genomics in paediatric acute lymphoblastic leukaemia[J]. Nat Rev Clin Oncol, 2019, 16(4): 227-240. doi: 10.1038/s41571-018-0136-6
[19] Nutt SL, Heavey B, Rolink AG, et al. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5[J]. Nature, 1999, 401(6753): 556-562. doi: 10.1038/44076
[20] Medvedovic J, Ebert A, Tagoh H, et al. Pax5: a master regulator of B cell development and leukemogenesis[J]. Adv Immunol, 2011, 111: 179-206.
[21] Revilla-I-Domingo R, Bilic I, Vilagos B, et al. The B-cell identity factor Pax5 regulates distinct transcriptional programmes in early and late B lymphopoiesis[J]. EMBO J, 2012, 31(14): 3130-3146. doi: 10.1038/emboj.2012.155
[22] Liu GJ, Cimmino L, Jude JG, et al. Pax5 loss imposes a reversible differentiation block in B-progenitor acute lymphoblastic leukemia[J]. Genes Dev, 2014, 28(12): 1337-1350. doi: 10.1101/gad.240416.114
[23] Zaliova M, Stuchly J, Winkowska L, et al. Genomic landscape of pediatric B-other acute lymphoblastic leukemia in a consecutive European cohort[J]. Haematologica, 2019, 104(7): 1396-1406. doi: 10.3324/haematol.2018.204974
[24] Dang J, Wei L, de Ridder J, et al. PAX5 is a tumor suppressor in mouse mutagenesis models of acute lymphoblastic leukemia[J]. Blood, 2015, 125(23): 3609-3617. doi: 10.1182/blood-2015-02-626127
[25] Duffield AS, Mullighan CG, Borowitz MJ. International Consensus Classification of acute lymphoblastic leukemia/lymphoma[J]. Virchows Arch, 2023, 482(1): 11-26. doi: 10.1007/s00428-022-03448-8
[26] Shah B, Mattison RJ, Abboud R, et al. Acute Lymphoblastic Leukemia, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2024, 22(8): 563-576. doi: 10.6004/jnccn.2024.0051
-

计量
- 文章访问数: 296
- 施引文献: 0



 下载:
下载: