-
摘要: 2022年同时发布了造血和淋巴组织肿瘤第5版WHO分类(WHO 2022)以及髓系肿瘤和急性白血病的国际分类共识(ICC 2022),其中均对髓系肿瘤包括骨髓增生异常综合征进行了更新。本文聚焦于骨髓增生异常综合征的关键更新内容,比较了WHO和ICC两种分类的不同之处,并结合临床实际运用进行相关解读。Abstract: In 2022, the 5th edition of the World Health Organization Classification of Hematolymphoid Tumors: Myeloid and Histiocytic/Dendritic Neoplasms(WHO 2022) and International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data(ICC 2022) were published simultaneously. Myeloid tumors, including myelodysplastic syndromes, were all updated. This article focuses on the key updates of myelodysplastic syndromes, compares the differences between WHO and ICC classification, and puts forward relevant interpretation based on clinical practice.
-
Key words:
- myelodysplastic syndrome /
- classification
-

-
[1] Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms[J]. Leukemia, 2022, 36(7): 1703-1719. doi: 10.1038/s41375-022-01613-1
[2] Arber DA, Orazi A, Hasserjian RP, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data[J]. Blood, 2022, 140(11): 1200-1228. doi: 10.1182/blood.2022015850
[3] Arber DA, Orazi A, Hasserjian RP, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia[J]. Blood, 2016, 127(20): 2391-2405. doi: 10.1182/blood-2016-03-643544
[4] Greenberg PL, Tuechler H, Schanz J, et al. Cytopenia levels for aiding establishment of the diagnosis of myelodysplastic syndromes[J]. Blood, 2016, 128(16): 2096-2097. doi: 10.1182/blood-2016-07-728766
[5] Estey E, Hasserjian RP, Döhner H. Distinguishing AML from MDS: a fixed blast percentage may no longer be optimal[J]. Blood, 2022, 139(3): 323-332. doi: 10.1182/blood.2021011304
[6] Chen X, Fromm JR, Naresh KN. "Blasts" in myeloid neoplasms-how do we define blasts and how do we incorporate them into diagnostic schema moving forward?[J]. Leukemia, 2022, 36(2): 327-332. doi: 10.1038/s41375-021-01498-6
[7] Malcovati L, Stevenson K, Papaemmanuil E, et al. SF3B1-mutant MDS as a distinct disease subtype: a proposal from the International Working Group for the Prognosis of MDS[J]. Blood, 2020, 136(2): 157-170. doi: 10.1182/blood.2020004850
[8] Platzbecker U, Della Porta MG, Santini V, et al. Efficacy and safety of luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive, transfusion-dependent, lower-risk myelodysplastic syndromes(COMMANDS): interim analysis of a phase 3, open-label, randomised controlled trial[J]. Lancet, 2023, 402(10399): 373-385. doi: 10.1016/S0140-6736(23)00874-7
[9] Lanino L, Restuccia F, Perego A, et al. Real-world efficacy and safety of luspatercept and predictive factors of response in patients with lower risk myelodysplastic syndromes with ring sideroblasts[J]. Am J Hematol, 2023, 98(8): E204-E208.
[10] Ma L, Luo Y, Jiang L, et al. The relation of SF3B1 mutation and intracellular iron in myelodysplastic syndrome with less than 5% bone marrow blasts[J]. Leuk Lymphoma, 2019, 60(5): 1179-1186. doi: 10.1080/10428194.2018.1520990
[11] Ma L, Liang B, Hu H, et al. A Novel Prognostic Scoring Model for Myelodysplastic Syndrome Patients With SF3B1 Mutation[J]. Front Oncol, 2022, 12: 905490. doi: 10.3389/fonc.2022.905490
[12] Haase D, Stevenson KE, Neuberg D, et al. TP53 mutation status divides myelodysplastic syndromes with complex karyotypes into distinct prognostic subgroups[J]. Leukemia, 2019, 33(7): 1747-1758. doi: 10.1038/s41375-018-0351-2
[13] Grob T, Al Hinai ASA, Sanders MA, et al. Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome[J]. Blood, 2022, 139(15): 2347-2354. doi: 10.1182/blood.2021014472
[14] Bernard E, Nannya Y, Hasserjian RP, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes[J]. Nat Med, 2020, 26(10): 1549-1556. doi: 10.1038/s41591-020-1008-z
[15] Ball S, Loghavi S, Zeidan AM. TP53-altered higher-risk myelodysplastic syndromes/neoplasms and acute myeloid leukemia: a distinct genetic entity with unique unmet needs[J]. Leuk Lymphoma, 2023, 64(3): 540-550. doi: 10.1080/10428194.2022.2136969
[16] Ren Y, Wang J, Zhang H, et al. TP53 mutations are associated with very complex karyotype and suggest poor prognosis in newly diagnosed myelodysplastic syndrome patients with monosomal karyotype[J]. Asia Pac J Clin Oncol, 2020, 16(3): 172-179. doi: 10.1111/ajco.13316
[17] Daver NG, Maiti A, Kadia TM, et al. TP53-Mutated Myelodysplastic Syndrome and Acute Myeloid Leukemia: Biology, Current Therapy, and Future Directions[J]. Cancer Discov, 2022, 12(11): 2516-2529. doi: 10.1158/2159-8290.CD-22-0332
[18] Jilg S, Reidel V, Müller-Thomas C, et al. Blockade of BCL-2 proteins efficiently induces apoptosis in progenitor cells of high-risk myelodysplastic syndromes patients[J]. Leukemia, 2016, 30(1): 112-123. doi: 10.1038/leu.2015.179
[19] Zeidan AM. Venetoclax and Azacitidine in the Treatment of Patients with Relapsed/Refractory Myelodysplastic Syndrome[J]. Blood, 2021, 138(Supplement 1): 537-537. doi: 10.1182/blood-2021-145646
[20] Sallman DA, Al Malki MM, Asch AS, et al. Magrolimab in Combination With Azacitidine in Patients With Higher-Risk Myelodysplastic Syndromes: Final Results of a Phase Ib Study[J]. J Clin Oncol, 2023, 41(15): 2815-2826. doi: 10.1200/JCO.22.01794
[21] Daver N, Boddu P, Garcia-Manero G, et al. Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes[J]. Leukemia, 2018, 32(5): 1094-1105. doi: 10.1038/s41375-018-0070-8
[22] Zeidan AM. Sabatolimab(MBG453) Combination Treatment Regimens for Patients(Pts)with Higher-Risk Myelodysplastic Syndromes(HR-MDS): The MDS Studies in the Stimulus Immuno-Myeloid Clinical Trial Program[J]. Blood, 2021, 138(Supplement 1): 4669-4669. doi: 10.1182/blood-2021-145626
[23] Loghavi S, Al-Ibraheemi A, Zuo Z, et al. TP53 overexpression is an independent adverse prognostic factor in de novo myelodysplastic syndromes with fibrosis[J]. Br J Haematol, 2015, 171(1): 91-99. doi: 10.1111/bjh.13529
[24] Buesche G, Teoman H, Wilczak W, et al. Marrow fibrosis predicts early fatal marrow failure in patients with myelodysplastic syndromes[J]. Leukemia, 2008, 22(2): 313-322. doi: 10.1038/sj.leu.2405030
[25] Zhao Y, Guo J, Zhao S, et al. Bone Marrow Fibrosis at Diagnosis and during the Course of Disease Is Associated with TP53 Mutations and Adverse Prognosis in Primary Myelodysplastic Syndrome[J]. Cancers(Basel), 2022.14(12): 2984.
[26] Zhang Y. Validation of the 5th Edition of the World Health Organization Classification of Myelodysplastic Neoplasms on 852 Consecutive De Novo Patients from a Single Institute[J]. Blood, 2022, 140(Supplement 1): 1343-1345. doi: 10.1182/blood-2022-160131
[27] Kobayashi T, Nannya Y, Ichikawa M, et al. A nationwide survey of hypoplastic myelodysplastic syndrome(a multicenter retrospective study)[J]. Am J Hematol, 2017, 92(12): 1324-1332. doi: 10.1002/ajh.24905
[28] Tuzuner N, Cox C, Rowe JM, et al. Hypocellular myelodysplastic syndromes(MDS): new proposals[J]. Br J Haematol, 1995, 91(3): 612-617. doi: 10.1111/j.1365-2141.1995.tb05356.x
[29] Nand S, Godwin JE. Hypoplastic myelodysplastic syndrome[J]. Cancer, 1988, 62(5): 958-964. doi: 10.1002/1097-0142(19880901)62:5<958::AID-CNCR2820620519>3.0.CO;2-P
[30] Huang TC, Ko BS, Tang JL, et al. Comparison of hypoplastic myelodysplastic syndrome(MDS)with normo-/hypercellular MDS by International Prognostic Scoring System, cytogenetic and genetic studies[J]. Leukemia, 2008, 22(3): 544-550. doi: 10.1038/sj.leu.2405076
[31] Bennett JM, Orazi A. Diagnostic criteria to distinguish hypocellular acute myeloid leukemia from hypocellular myelodysplastic syndromes and aplastic anemia: recommendations for a standardized approach[J]. Haematologica, 2009, 94(2): 264-268. doi: 10.3324/haematol.13755
[32] Bono E, McLornan D, Travaglino E, et al. Clinical, histopathological and molecular characterization of hypoplastic myelodysplastic syndrome[J]. Leukemia, 2019, 33(10): 2495-2505. doi: 10.1038/s41375-019-0457-1
[33] Calabretto G, Attardi E, Teramo A, et al. Hypocellular myelodysplastic syndromes(h-MDS): from clinical description to immunological characterization in the Italian multi-center experience[J]. Leukemia, 2022, 36(7): 1947-1950. doi: 10.1038/s41375-022-01592-3
[34] Nazha A, Seastone D, Radivoyevitch T, et al. Genomic patterns associated with hypoplastic compared to hyperplastic myelodysplastic syndromes[J]. Haematologica, 2015, 100(11): e434-e437. doi: 10.3324/haematol.2015.130112
[35] Baidoun F, Chen D, Patnaik M, et al. Clinical outcome of patients diagnosed with myelodysplastic syndrome-unclassifiable(MDS-U): single center experience[J]. Leuk Lymphoma, 2019, 60(10): 2483-2487. doi: 10.1080/10428194.2019.1581930
[36] Font P, Loscertales J, Soto C, et al. Interobserver variance in myelodysplastic syndromes with less than 5% bone marrow blasts: unilineage vs. multilineage dysplasia and reproducibility of the threshold of 2% blasts[J]. Ann Hematol, 2015, 94(4): 565-573. doi: 10.1007/s00277-014-2252-4
[37] Maassen A, Strupp C, Giagounidis A, et al. Validation and proposals for a refinement of the WHO 2008 classification of myelodysplastic syndromes without excess of blasts[J]. Leuk Res, 2013, 37(1): 64-70. doi: 10.1016/j.leukres.2012.09.021
-

| 引用本文: | 佟红艳. 骨髓增生异常综合征分类更新及运用解读[J]. 临床血液学杂志, 2023, 36(11): 773-778. doi: 10.13201/j.issn.1004-2806.2023.11.003 |
| Citation: | TONG Hongyan. Classification update and application interpretation of myelodysplastic syndromes[J]. J Clin Hematol, 2023, 36(11): 773-778. doi: 10.13201/j.issn.1004-2806.2023.11.003 |
- Figure 1.



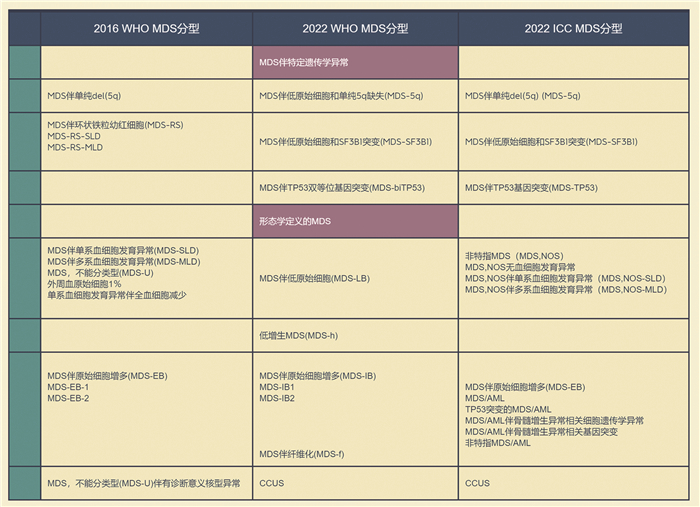
 下载:
下载: